Muscle memory is a phenomenon that has already been discussed in this blog, by myself at the beginning of 2019. This article rewrites and updates the previous one with new ones evidence and a more didactic approach than the previous one.
Do you want to know what muscle memory is? Don’t miss it!
What is it
Muscle memory is a concept coined by athletes who felt that after a break (due to injury or other reasons) in which they lost muscle mass, they were able to recover their muscle volume previous much faster than when they started.

Figure I. Changes by Kevin Levrone. First image from the 90s, second image 2008, third image 2017.
This empirical phenomenon has been evaluated on several occasions in scientific tests, and it has been proven that it certainly exists, but how is it possible?
Muscle-memory, nothing new on the horizon?
The body is efficient and one of the biological principles that govern life is that efficiency.
The body adapts to the increasing demands we place on it to become more efficient , for example:
When we move to a place that is much higher above sea level than we normally find, the partial pressure of oxygen decreases and our body increases erythropoiesis to compensate for the decrease action of the absorption of oxygen from the environment and prevention of tissue hypoxia.
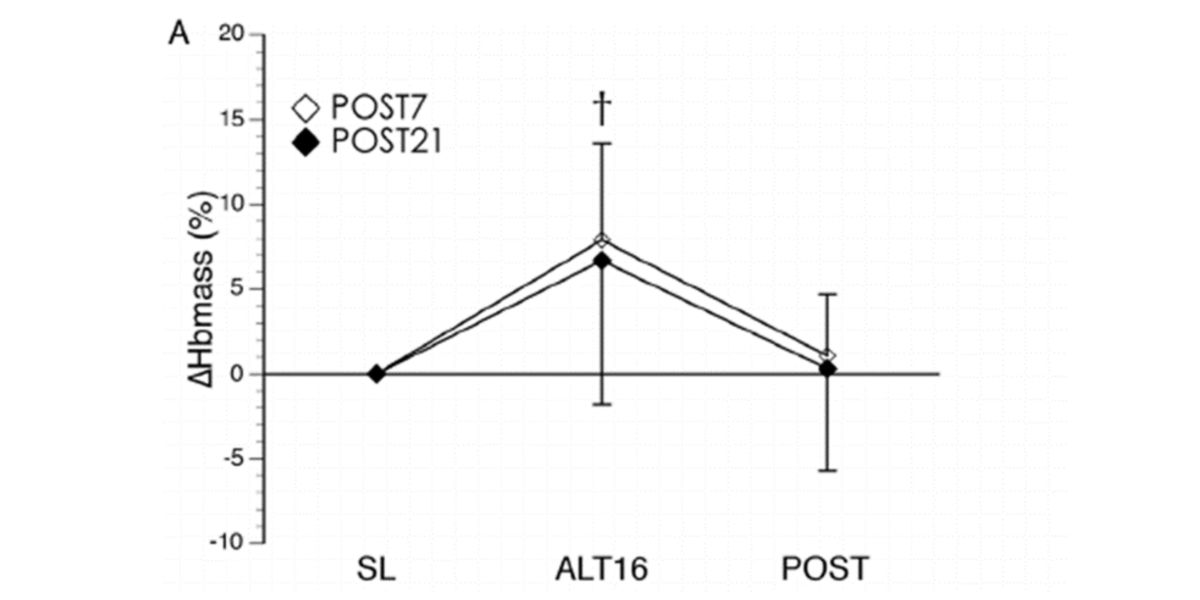
Figure II. Changes in hemoglobin concentrations after 16 days of acclimatization to + 5000 m altitude and after returning to the original level for 7 and 21 days.
When we return to our usual place, the concentrations of hemoglobin return to their initial state, because we no longer need that adaptation .
The same happens with muscle tissue :
Given the increasing demands for strength production, and taking into account that muscle size is one of the major determinants of it, our tissues adapt to the damage produced by receiving new nuclei that can help to repair muscle fibers.
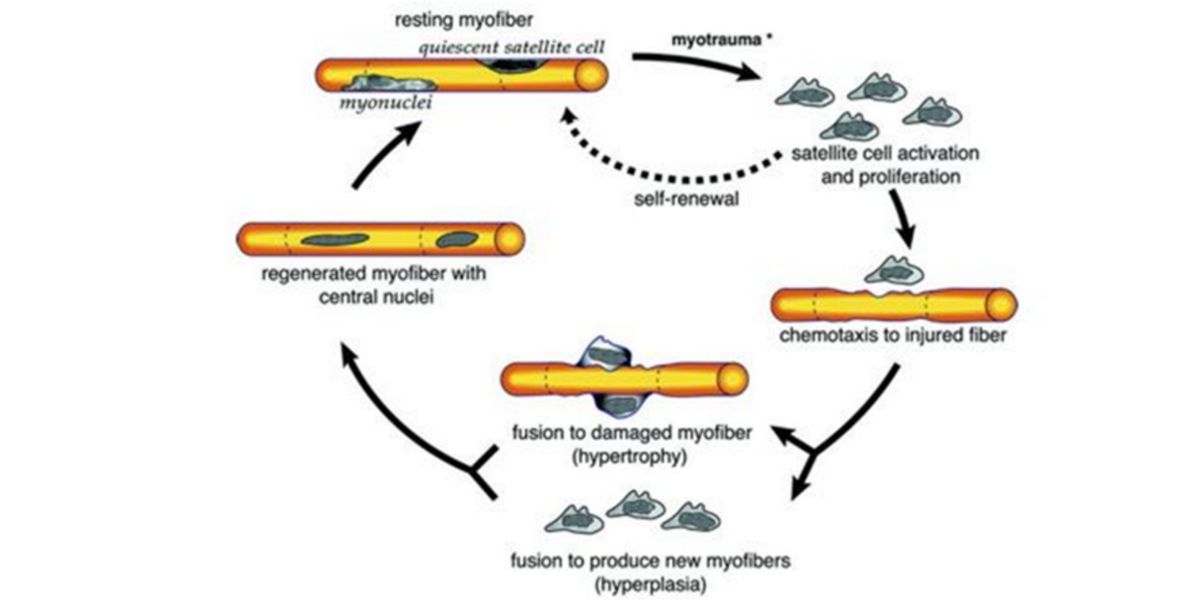
Figure III. Process of proliferation, chemotaxis and fusion of satellite cells into damaged muscle fiber.
To understand this process, it is important to understand that muscle cells are polynuclear cells and that each of these nuclei controls the transcriptional activity of a certain area of the muscle (called nuclear domain ), let’s imagine it like this:
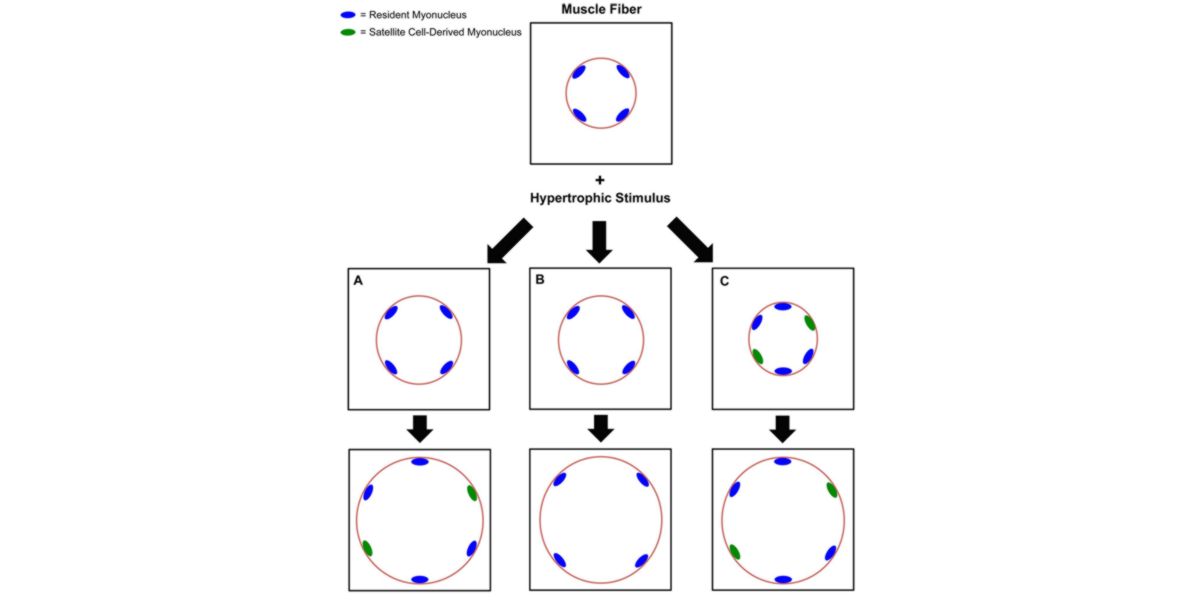
Figure IV. 3 possible phenotypes of muscle hypertrophy after a stimulus (training).
As if it were a construction on a circular perimeter, and each blue dot is a worker who is in charge of building a certain area, when they start working ( we train ), the workers get tired ( muscle damage ), and when they realize that there is a lot of work they call more collaborators.
In other words, new workers who have not worked in this building but who come to help (green dots which are cores given by satellite cells), since there are more workers, they can work faster, they endure more work ( because everyone has a smaller area) and can make a building bigger and more beautiful.
However, this is not unequivocal and it can happen that 4 workers (hypothesis in the center of the image) are used to make the construction.
Perfect, this is one of the principles of hypertrophy , which explains that each nucleus controls the protein synthesis (transcriptomic) activity of an area of the cell, the more myionuclei, the greater the ability to tolerate a greater impact of the training stimulus and the stronger the next (net) protein synthesis, i.e. the more it will hypertrophy.
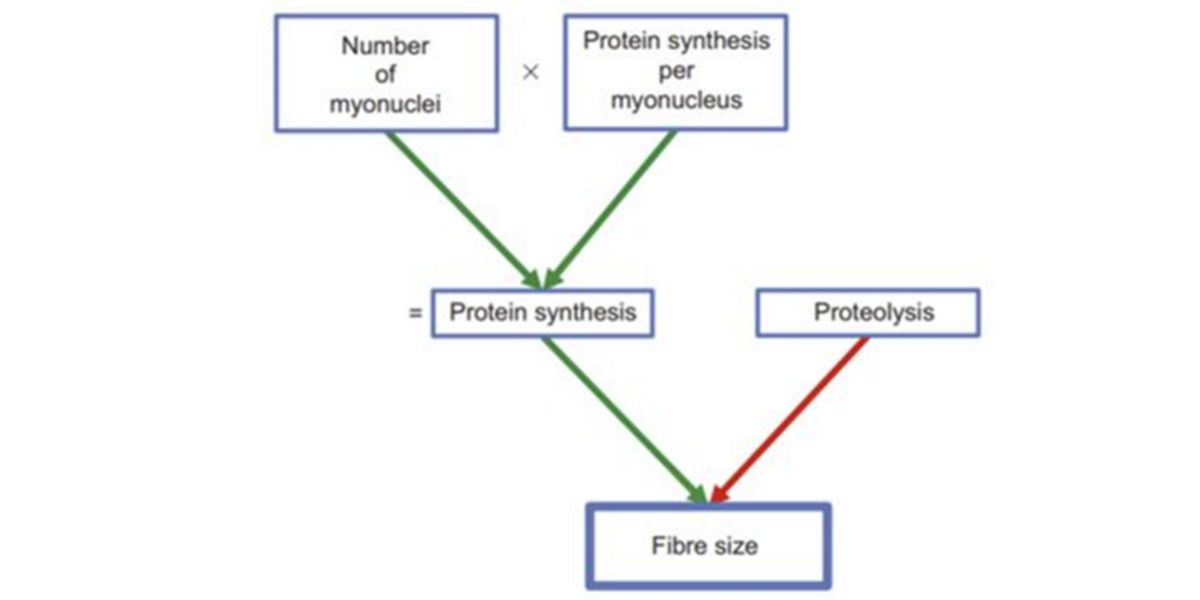
Figure V. Factors that regulate fiber size (hypertrophy / atrophy).
This has been demonstrated in several tests, where it has been shown that the size of the muscle fibers maintains an almost perfect correlation with the number of myonuclei
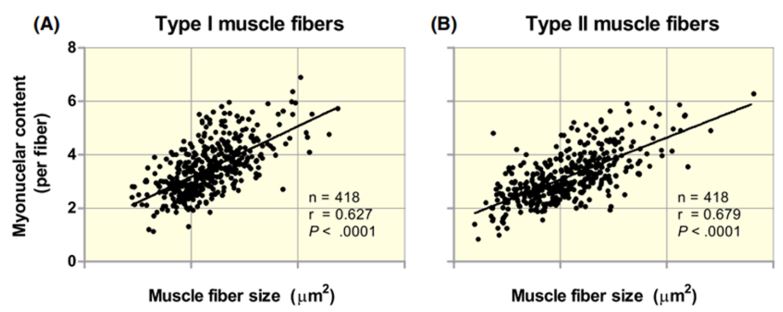
Figure VI. Relationship between the size of muscle fibers and the number of myonuclei per fiber.
An example of muscle-memory
Staron et al., (1991) conducted a very interesting study on healthy women in which they showed that muscle memory really existed.
The following image shows it perfectly :
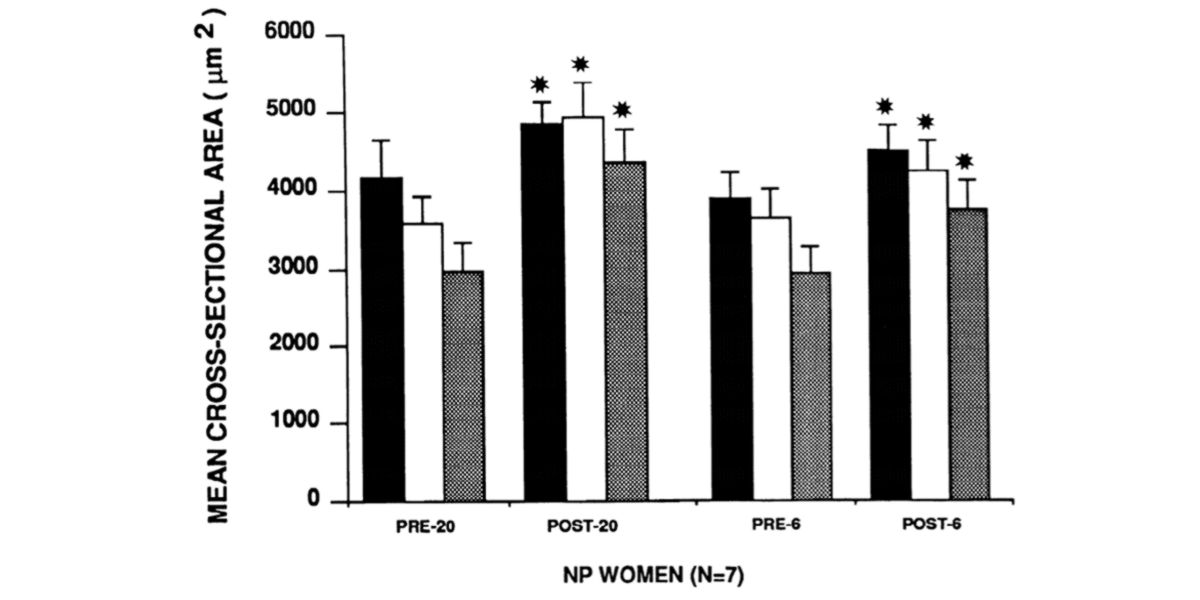
Figure VII. Changes in the size of type I (black bars) and type II fibers (it does not matter because the classification currently used is obsolete), before and after training and re-training.
Women in their initial state (Pre-20) underwent 20 weeks of strength training (Post-20) and, as noted, increased the size of all their muscle fiber types; Subsequently they were subjected to 30-32 weeks of de-training, where as you can see their muscle fibers, especially the more glycolytic ones (type II), decreased in size (pre-6), subsequently they were subjected to a re-training program. 6-week (post-6) training with which they recovered the previous state.
So, something happens, there is something so that in 6 weeks we can regain the lost muscle mass that we had taken 20 weeks to gain, right?
Theory of myonuclear-permanence
In the past it was believed that during the period of relaxation, the fused myonuclei muscle cells underwent an apoptotic process during training , that is, since they were no longer needed, they were lost.
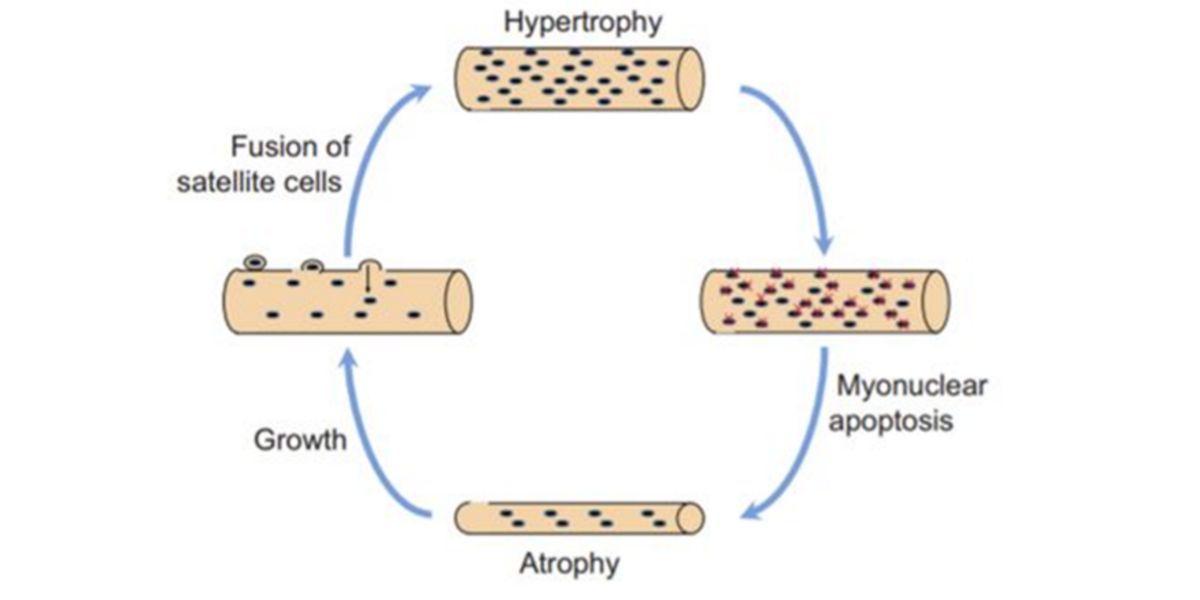
Figure VIII. Original hypothesis of the de-training and re-training process.
Subsequently, it was observed in in vivo animal tests that de-training did not reduce the number of myonuclei:
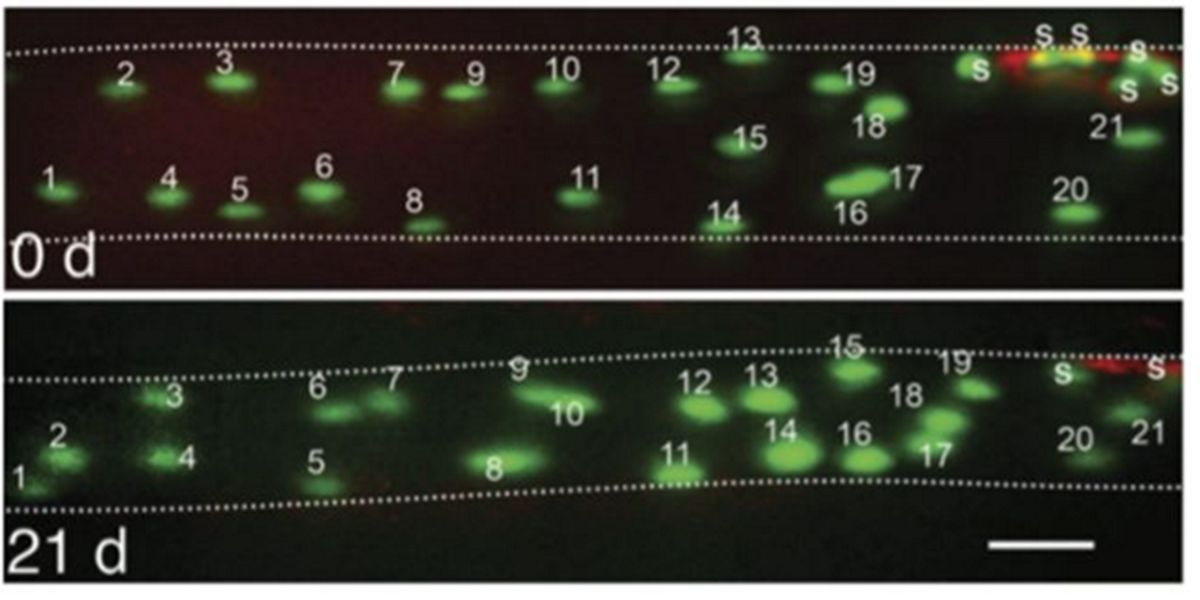
Figure IX. In vivo image of the number of myonuclei before and after 21 days of distension in a muscle cell from an animal model.
This gave a rationale for the muscle memory theory : we gain muscle mass faster because we don’t have to invest time in the satellite cell migration process to increase the number of myonuclei, that’s all.
Figure X . Hypothesis of the dominance / permanence of myonuclei.
And so the theory appeared, where it is explained that muscle fibers decrease their size, but not the number of nuclei they have acquired , so that when the demands for force production increase by again, the fabric will recover your previous training volume without the great efforts initially required.
This theory would be perfect, in fact it was the one I raised as an explanation in the first article, except that not is validated in humans , and in fact the only study currently existing in live humans (Psilander et al., 2019) was unable to find changes in the content of the myionucleus during the early period of training, de-training or re-training.
My usual bad luck!
Furthermore, animal models are very heterogeneous , this is due to the variety of analytical methods used to discriminate true myonuclei from other cellular structures (such as fibroblasts or satellite cells) which can skew the results.
In the review by Snijders et al. (2019) you can see all the details of this critical analysis.
What I mean is that according to biological teleology , the conflicting results in rodents and the absence of tests that demonstrate this theory in humans (while there are tests showing that myionuclei content is not stable throughout life):
It seems unlikely, or at least premature, to state that this is the reason for muscle memory.
Epigenetic theory
In the first article I wrote I talked about it, but I didn’t enter into this possibility given the complexity of the topic, even so, currently it is (perhaps) the theory that has more strength to explain, at least, part of the reasons underlying muscle memory in humans.
Surely you have ever heard that smoking increases the risk of lung cancer, this is a induction process epigenetics , because from exposure to the pollutants present in cigarette smoke, the body undergoes a series of genomic changes that lead you to develop a disease.
E if strength training produces genomic changes? Of course it does!
Weight training is able to modulate the transcriptomic response of muscle tissue cells , among other things, to increase protein synthesis and become bigger and exert more strength (as I explained earlier).
Some of these genetic changes only occur with exposure to an intense session of training!
Other genes require exposure to the stimulus ( training ) for a longer time to undergo changes in their activation ( methylation ), while others may not be altered, or we haven’t been able to prove it yet.
These changes in some genes modulate the response of a large number of signaling pathways.
Indeed, the study by Seaborne et al., (2018) shows that the hypomethylation of certain sets of genes assessed induced by resistance training is associated with an increase in transcriptional activity of the path PI3K-AKT-mTORC1.
Or:
The regulation of certain genes through exercise partly explains why we grow muscularly, and also goes beyond the roots of the myonucleus fusion process that we explained earlier.
The article by Seaorne et al. (2018) showed that when a group of men underwent a 7-week training program, their muscle mass increased.
By the time they stopped training for 7 weeks, lost practically everything and when they went back to training for another 7 weeks they recovered and surpassed their previous best form.
The authors evaluated the genetic changes that occurred during the study and observed a very interesting trend for large numbers of evaluated CpGs (regions of certain genes) to be hypomethylated, something that presumably increases gene activity.
This remained practically intact during the distension period, and increased significantly during the recovery period. -training , reaching 18,816 hypomethylated CpGs.
The authors observed that there were groups of genes (and specific genes) that showed a tendency to vary their degree of methylation to depending on whether the subjects were trained or not (cluster A):
While others varied sharply before training and then normalized (Cluster D) , others had a late response (Cluster C) and others changed before training exposure and were already maintained throughout the process (Cluster B).
Since the behavior of all loci is not identical (can be seen in the image colormap), the authors determined that some tendencies to hypo- or hyper-methylation of genomic clusters were associated with hypertrophic skeletal muscle response , which is why the study subjects regained muscle mass so quickly.
That is, the authors accused that the changes that formation induced in the activity of genes (and that some of them such as RPL35a / UBR5 / SETDF3 and PLA2G16 were particularly sensitive to this) unequivocally related to increased muscle volume and explained the reasons for the greater effects through re-training.
A summary of everything
Although muscle memory undoubtedly exists in animal models and appears (at least it is seen in several studies) to exist in humans, the reasons why it occurs are all. but clear.
Despite the fact that in rodents the theory of myonuclear domain (permanence of myonuclei) may be a real possibility under certain circumstances, it is necessary to clarify exactly which ones through the standardization of techniques used to avoid errors in the results and then see and whether it is replicable in humans.
We also need to evaluate the epigenetic theory (which is currently the most valid in humans) on much larger samples, as the study by Seaorne et al. (2018) uses only 8 adults, and all genome-wide methylations and increases in transcriptional activity must be sought, as well as investigating the effects they have on the organism and the extent of these, to determine if the relationship is really causal.






